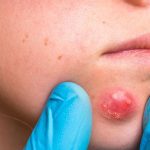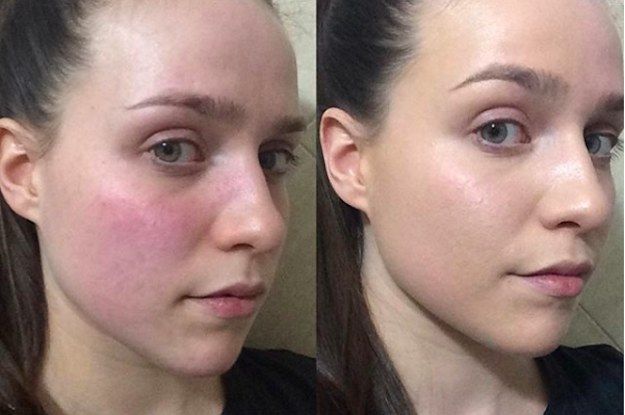
Some words on a product like
– Hypoallergenic
– For Sensitive Skin
Which means for sensitive skin or safe for sensitive skin.
WHY ??
Why can these products apply this to their products?
Does this claim really prove that it is for people with sensitive skin?
History
197 In 1974, the FDA recommended that any product be labeled as Hypoallergenic, with tests that confirm that the product is for sensitive or safe skin. Reactions compared to common products on the market, but unfortunately this proposal was rejected because the product companies say that testing is expensive and difficult to do because each person is allergic to different substances. If the product claims like this, they may be sued by the consumer if their product causes an allergy to any customer. Finally, in 1975, the FDA rescinded the proposal.
What is Hypoallergenic
The word Hypoallergenic or For Sensitive Skin is just a word for a product that is sold separately, the FDA has made it clear that this word is not a word that is safer than products without this word. In addition, the product or company can apply without any experience or certification, so they want to apply or not, depending on the company of the product. So if you have sensitive skin, the fate of your skin is on your hands, because as I mentioned above, each person is allergic to different substances, it is an individual problem that you yourself need to be careful because you need to know That the product on the market is a product that most people need, not for one or two people.
Consumers concerned about cosmetic allergies should understand one of the basics: “non-alcoholic” cosmetics, only cosmetics that can guarantee that they never produce an allergic reaction.
But are some cosmetics less likely to react negatively than competing products!!
In other words, the key ingredients in cosmetics, called “hypoallergenic”, are the same as those used in other cosmetics that are sold for the same purpose. Over the years, some cosmetics contain deadly ingredients that have the potential to cause side effects. But these ingredients are no longer used. The Food and Drug Administration is unaware that there are no scientific studies showing that cosmetics or products cause similar reactions, causing fewer adverse reactions than normal product competition.
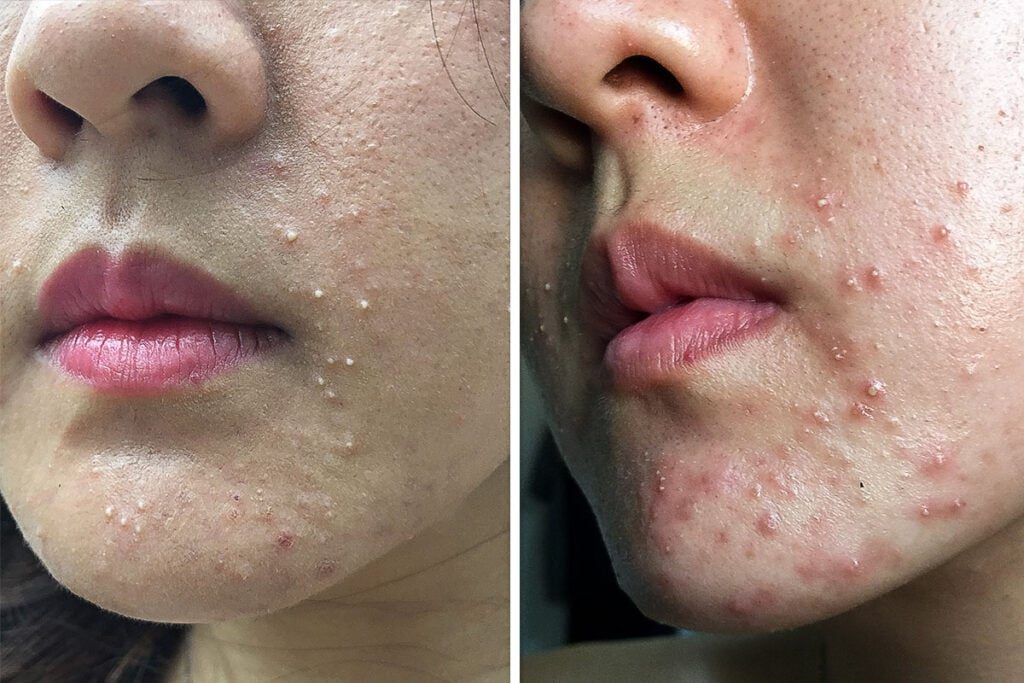
The court determined that the definition of FDA Of the word “hypoallergenic” is irrational because the service business does not indicate that people who use the product or service perceive the word “apoloclane” in the way described in the regulations.
As a result of the decision, manufacturers may continue to label and advertise their cosmetics as “epolin” or make almost identical claims without any supporting material indicating anything. Those who use the product or service will have no promise that such claims are valid.
However, cosmetic users who know they are more likely to have adverse and unpleasant reactions to certain ingredients can take steps to protect themselves. FDA regulations now require that ingredients used in cosmetics be listed on the product label so that those who use the product or service can avoid the substance that is causing them problems
 Login as
Login as